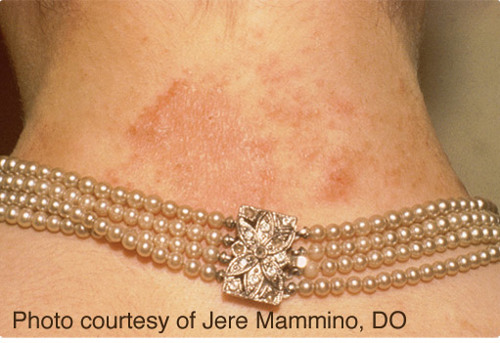
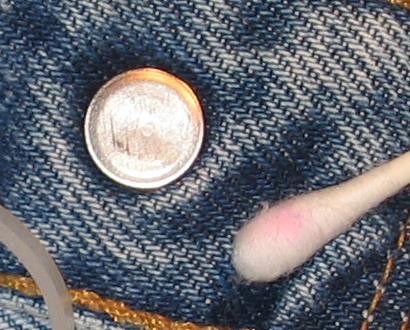
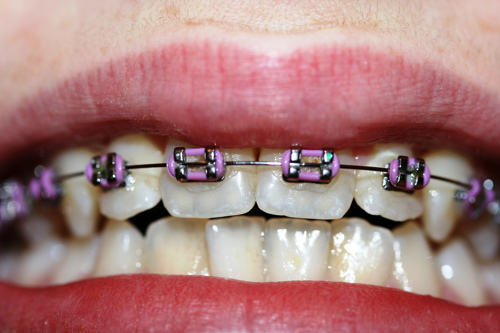
Nickel is one of the most common causes of allergic contact dermatitis (ACD). Nickel sensitization is the condition of being allergic to nickel. Nickel ACD reactions occur in nickel-sensitized (or nickel allergic) individuals. The reason for the relatively high prevalence of nickel sensitization is due to the use of nickel-releasing consumer items that come in direct and prolonged contact with the skin. Although exposure may occur through some occupational settings generally associated with soluble nickel salts, the marked prevalence of nickel sensitization in the general population is primarily due to consumer dermal exposure to nickel released from nickel metal in plated articles or some alloys (e.g., in jewelry, watches, eyeglasses). Nickel sensitization and nickel ACD require prolonged exposure of the immune system to nickel absorbed through the skin. Once sensitized, exposure to a sufficient amount of nickel over an extended time causes nickel ACD reactions by the immune system, called elicitation. The release of nickel ions is responsible for causing nickel sensitization and nickel ACD, which are threshold effects (requiring release of ions above a specific amount to cause a reaction). Alloys such as many stainless steels contain nickel but do not release a sufficient amount of nickel-ions to cause an individual to become nickel sensitized or have a nickel ACD reaction if they are already nickel-sensitized.
Exposure to nickel ions through inhalation and oral exposure has not been shown to induce nickel-sensitization in non-nickel-sensitized individuals. Rather, oral exposure in some cases has been demonstrated to reduce susceptibility or even result in immunotolerance to dermal nickel sensitization.
Legislation in the European Union has been put in place to decrease the consumer dermal exposure to nickel-releasing articles intended to come in direct and prolonged skin contact, to reduce the prevalence of nickel-sensitized individuals and the number of nickel ACD reactions in already nickel-sensitized individuals. Occupational exposure is being reduced through risk management measures within the workplaces. The nickel industry supports and encourages these practices to reduce and minimize nickel allergic contact dermatitis.
last revision: June 2016
© 2016, NiPERA Inc.