In addition to their role in the production of biodiesel, renewable diesel and SAF (sustainable aviation fuel), nickel catalysts are used for water electrolysers for hydrogen production. Hydrogen can then be burned directly, used in fuel cells, used to hydrotreat other renewable fuels, or be converted to another fuel such as methanol or ammonia. Hydrogen can also be used in the net-zero production of other chemicals, or for production of steel from iron ore.
Nickel catalysts - the many routes to decarbonising transport

Nickel-based catalysts are key to supplying energy to power our transportation of goods and people, whether by land, sea, or air.
Electroysers and fuel cells
Electrolysers split water into oxygen and hydrogen. A fuel cell is an electrolyser in reverse, combining oxygen and hydrogen into water to produce electricity. When produced using renewable energy, this is called “green” hydrogen, and the International Energy Agency predicts that demand for it will explode over the next 25 years.
There are four types of electrolysers available today:
- Polymer electrolyte membrane (also known as proton-exchange membrane, PEM),
- Alkaline water electrolyser (AWE),
- Anion exchange membrane (AEM),
- Solid-oxide electrolyser cell (SOEC).
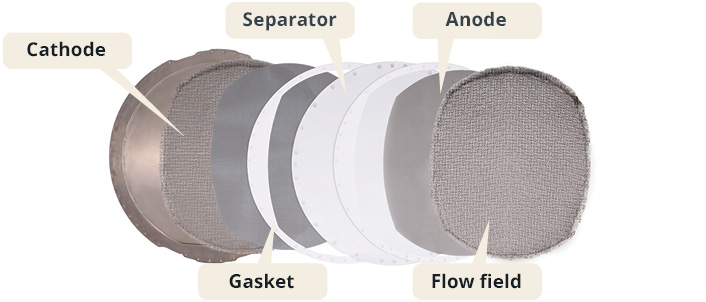
While PEM electrolysers are the most efficient, they require the use of platinum and iridium as electrocatalysts, while the other three technologies use nickel-based electrocatalysts. Of the nickel-containing types, AWEs are the most commercially advanced. In AWEs, nickel is found in the anode, the cathode, and in the flow field components of the electrolyser cell.
The flow field consists of a loose nickel mesh, to allow for easy transport of electrolyte and evolved gases. The anode is typically an engineered nickel mesh consisting of sintered fibres, while the cathode is a similar nickel fibre mesh coated with a catalyst. These catalysts are proprietary to the supplier, but NiFeOx is commonly used.
Electrolysers
-
AEM: Anion exchange membrane
-
AWE: Alkaline water electrolyser
-
PEM: Polymer electrolyte membrane/Proton-exchange membrane
-
SOEC: Solid-oxide electrolyser cell
Pros and cons of different catalysts
The relatively low capital cost of AWEs has led to their increasing use, but in order to minimise production costs and meet the demand for renewable electricity, AEMs and SOECs are being introduced.
AEMs have the benefit of higher electrical efficiency compared to AWEs, while using similar materials, and so are often thought of as an intermediate between AWEs and PEMs. Like AWEs, AEMs use nickel meshes to control flow and to deliver current, and also use nickel catalysts. In contrast to the relatively simple catalysts used in AWEs, AEM catalysts tend to be more highly engineered. Proposed anode catalysts showing high performance include NiFe double layered hydroxides supported on carbon paper and NiCoSe alloys supported on Ni foam. Cathode catalysts tend to be NiFe and NiCo alloys supported on Ni mesh.
SOECs operate at very high temperatures (>600 °C) and offer high efficiency with the ability to integrate waste heat into industrial applications. In SOECs, the electrocatalyst, often Ni metal particles, is supported directly on the electrolyte, which is a metal oxide that becomes conductive to ions at the high operating temperature.
Irreplaceable nickel
These are just examples of the role that catalysts and other components using nickel will play in a decarbonised future. Nickel’s relatively low cost, inertness to caustic substances, and specific performance features in electrolysers make it an irreplaceable part of these emerging technologies.
This article was first published in our Nickel Magazine VOL 38-2, in Summer 2023.


