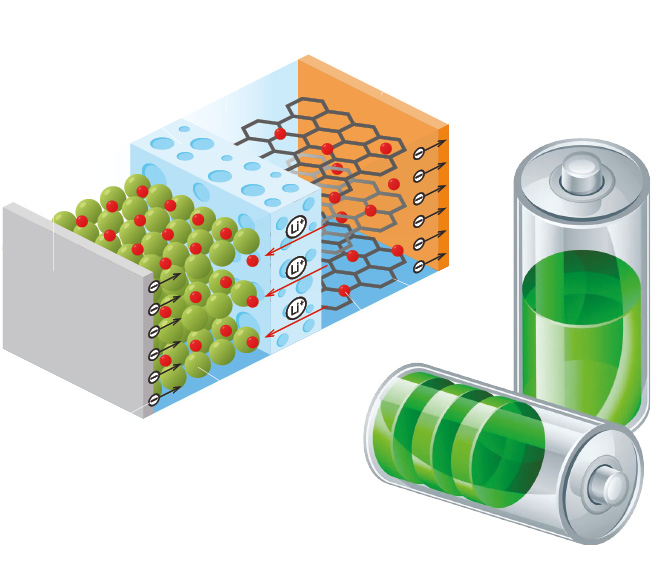
The battery
A battery converts chemical energy into electric energy. It powers devices, such as smartphones, portable power tools and cars. Batteries come in various chemistries, shapes and sizes depending on the size and power requirements of the device being powered. It consists of one or more electrochemical cells, which is comprised of two electrodes – an anode and a cathode – and an electrolyte. When the two electrodes are linked by an electrical pathway, electrons flow from the anode and
deliver energy to an external device.