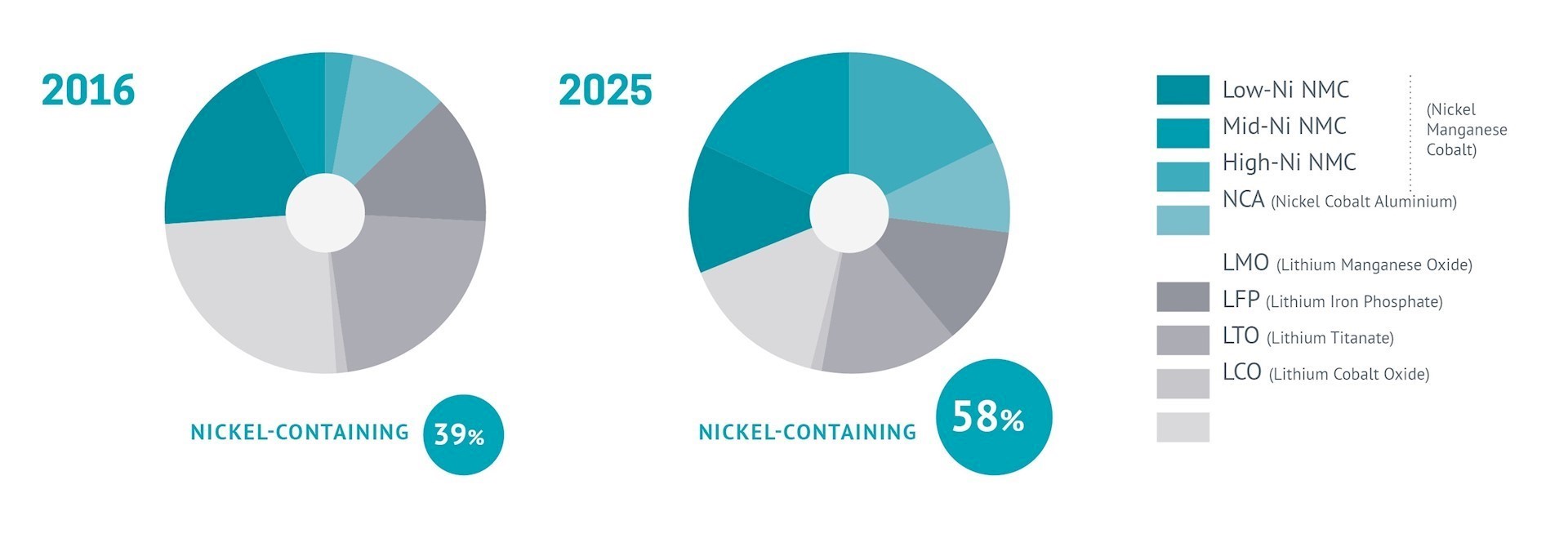
Nickel (Ni) has long been widely used in batteries, most commonly in nickel cadmium (NiCd) and in the longer-lasting nickel metal hydride (NiMH) rechargeable batteries, which came to the fore in the 1980s. Their adoption in power tools and early digital cameras revealed the potential for portable devices, changing expectations of how we work and live. The mid-1990s saw the first significant use of NiMH batteries in vehicles in the Toyota Prius. Around the same time, the first commercial applications for Li-ion batteries emerged, initially in camcorders and eventually finding their way into smartphones, laptops and the numerous other portable devices we now take for granted.
The major advantage of using nickel in batteries is that it helps deliver higher energy density and greater storage capacity at a lower cost. Further advances in nickel-containing battery technology mean it is set for an increasing role in energy storage systems, helping make the cost of each kWh of battery storage more competitive. It is making energy production from intermittent renewable energy sources such as wind and solar replace fossil fuels more viable.