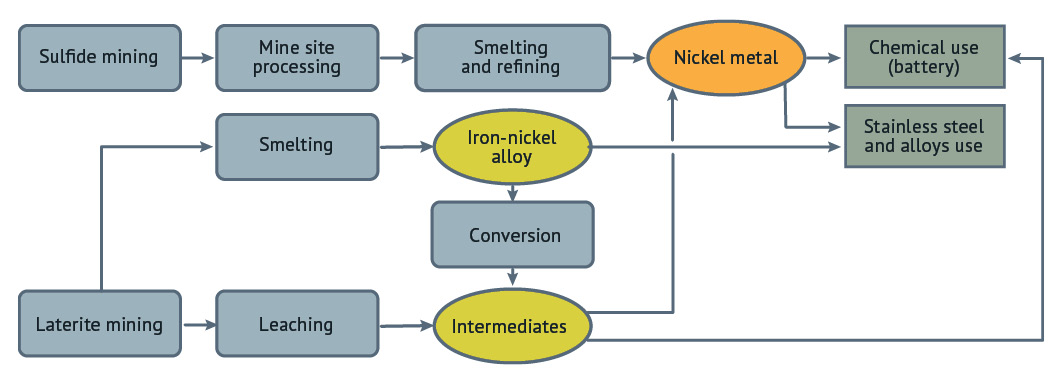
Nickel is primarily used in two areas today: stainless steel and other alloys, and chemical uses including batteries. Stainless steel is the largest part of the nickel market, while batteries are the fastest growing.
To meet the global nickel demand, nickel is produced from two main sources: fresh ores and recycled materials. Recycling (whether stainless steel and nickel alloys or batteries) is an important part of the nickel value chain. The very high recylability of nickel is a critical part of its sustainability profile, but most of the nickel market is focused on the fresh material put into the supply chain, now about 3 million tonnes/year.